Learn more about our current research
Lab Research Overview Video
Atmospheric Chemistry Video

Chemistry that drive toxic emissions from e-cigarette vaping

Our research on nicotine based e-cigarettes focuses on using advanced analytical chemistry tools to evaluate the toxic emission profile of vaping devices. We compare the chemical composition of the vape liquid before vaping and the aerosol emissions after the vaping process. Our current projects focus on the chemistry that produces toxic organic compounds, reactive oxidants, and metal species in vape aerosols from disposable e-cigarette products. We do stereospecific analysis of nicotine and molecular-level analysis of flavor chemicals and toxic carbonyls formed from the thermal degradation process during vaping.
This project uses GC-MS, LC-HRMS (Orbitrap-based high resolution mass spectrometry), UV/Vis spectroscopy, and others as needed while developing new and innovative analytical methods and sampling techniques that are suitable for a diverse set of devices and analytes.
- This research is funded by the Tobacco Related Disease Research Program.
Chemistry of Cannabis Vaping: Toxicants, Cannabinoids, and Transformation Pathways
Our research focuses on a complex characterization of the aerosol produced from cannabinoid-based e-cigarettes. This includes examining toxicant formation, cannabinoid dose, and the chemical transformation pathways that occur during the vaping process. We evaluate a wide variety of e-liquids and distillates, including THC isomers, CBD, and their O-acetate derivatives. Toxicants, such ketene and carbonyls, are quantified using LC-HRMS, while cannabinoid doses are measured with GC-MS. We have a current Schedule 1 Researcher License for working with marijuana and delta-9 THC.
Our analysis has includes well controlled systems and commercially available cannabis-based e-cigarettes. A variety of analytical techniques, including GC-MS, LC-HRMS, and GC-FID are utilized to detect cannabinoids and volatile organic compounds in aerosols under typical vaping conditions. We strive to develop adaptable sampling techniques that meet the requirements of a rapidly evolving cannabis vaping market.
- This research is funded by the Department of Cannabis Control.

Can wildfire smoke affect radical oxidant formation in atmospheric aerosols?

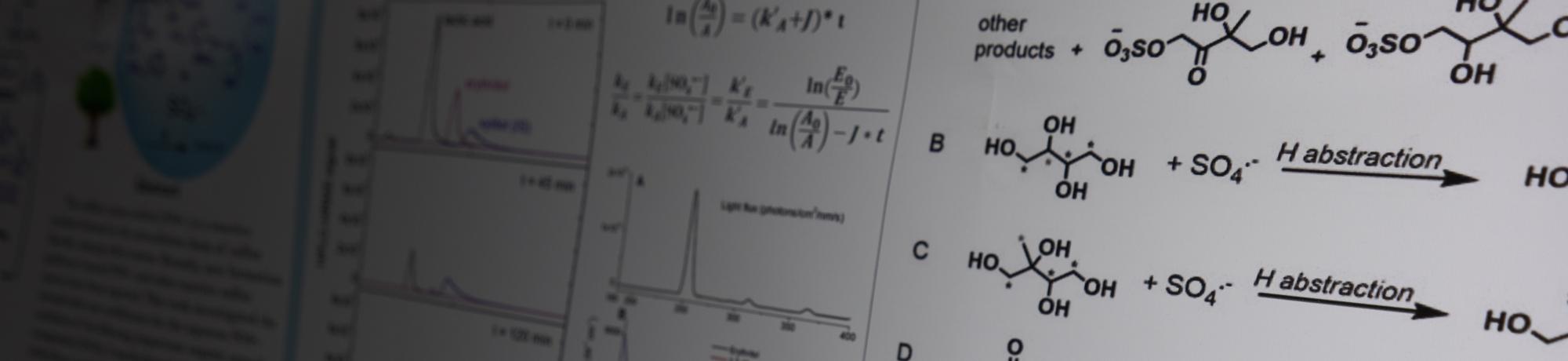
Particles in the atmosphere are mixtures of organic and inorganic compounds. Increasingly, the inorganic ammonium sulfate (AS) particles that are prevalent in the atmosphere will be mixed with organic brown carbon (BrC) from wildfires. BrC contain light absorbing compounds that can form triplet excited states (3C*) when irradiated, which can photosensitize reactions. We are studying how irradiated BrC compounds can initiate or enhance photochemistry from AS particles, producing reactive sulfate radicals (SO4∙−) that degrade organic molecules.
We use a chemical trap to capture the sulfate radical as an organosulfate (OS) for high-resolution mass spec analysis and trace the reactions back to sulfate radical chemistry.

- This research is funded by the NSF.
Measuring Photooxidants in Particulate Matter
Photooxidants in particle liquid water (e.g., OH, 1O2, 3C*, NO3, SO4) are important sinks of organics in the particles. This affects the particle composition and mass of the particles, which changes the particle's air quality or climate properties. Concentrations of oxidants in particles have never been measured due to the fact that oxidants are very reactive and their concentrations are highly trace, so measuring their concentrations on microscopic particulate matter (less than 1 micrometer in diameter) comes with highly significant challenges. As modeled values vary by three to four orders of magnitude, any measurements will provide much needed constraints. We are developing a method to measure oxidants in the PLW of particles using using chemical probes in an atmospheric chamber. We track the decay of the probes with time to follow the oxidation reaction. We aim to produce proof of concept results in our model system, and apply the method to atmospheric particulate matter.
ICARUS - The Atmospheric Chamber Database
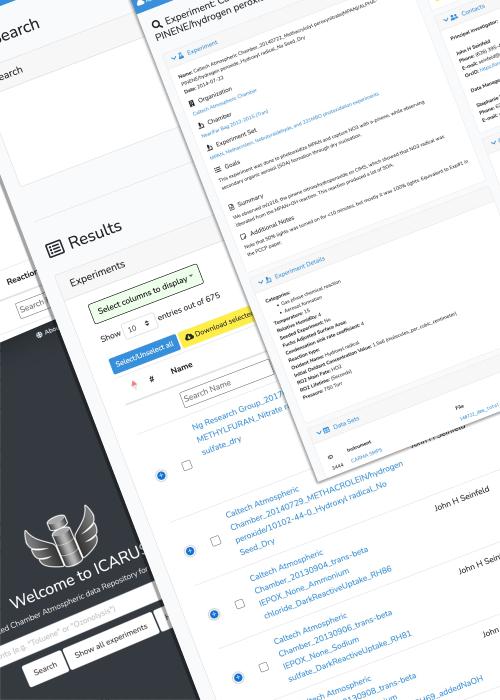
Our lab led the development of the first atmospheric chamber database in the United States called ICARUS (https://icarus.ucdavis.edu). Atmospheric chambers have been critcial research tools since the 1950's, producing data that are used to develop model mechanisms of air pollution and climate, and further the fundamental knowledge of chemistry and physics in the atmosphere. However, there was no central hub to store, categorize, access, and share the data produced from the decades of atmospheric chamber research. ICARUS was borne from an NSF-funded collaborative effort between UC Davis and several other institutions (Caltech, NCAR, Carnegie Mellon, UC Irvine, UC Riverside, Georgia Tech, University of Colorado, University of Texas). The database is operational and available to host any atmospheric chamber research results.
- This research is funded by the NSF.
